What is Mounjaro?
Mounjaro (tirzepatide) is a prescription injectable medication that has emerged as a promising treatment option for weight loss/ weight management and for adults with type 2 diabetes mellitus. This prescription injectable medication offers a versatile approach, functioning in two primary capacities:
1. Management of Type 2 Diabetes:
- Monotherapy: Mounjaro can be prescribed as the sole diabetic medication for patients who are unable to tolerate metformin, a commonly used first-line treatment.
- Combination Therapy: For patients whose blood sugar levels remain uncontrolled with existing medications, Mounjaro can be effectively combined with other oral therapies and/or injectable insulin.
2. Weight Management in Adults:
In combination with a reduced-calorie diet and increased physical activity, Mounjaro for weight loss and weight management is approved in specific adult populations:
- Individuals with Obesity (BMI ≥ 30 kg/m²): Mounjaro can be a valuable tool for weight management in patients classified as obese based on Body Mass Index (BMI).
- Overweight Adults (BMI 27-30 kg/m²) with Weight-Related Comorbidities: Mounjaro may be a suitable option for overweight adults (BMI 27-30 kg/m²) who are struggling with weight management and have additional weight-related health concerns. These concerns may include prediabetes, type 2 diabetes, high blood pressure, abnormal cholesterol levels, sleep apnea, or a history of cardiovascular events.
When was Mounjaro launched in the UK?
- November 2023: The Medicines and Healthcare products Regulatory Agency (MHRA) granted approval for Mounjaro (tirzepatide).
- January 2024: MHRA approved Mounjaro Kwikpen to treat adults with type 2 diabetes and for weight management in adult patients.
- February 2024: Following approval, Mounjaro became available for purchase with a prescription at UK pharmacies.
How does Mounjaro work?
Mounjaro works through an unique mechanism targeting two key gut hormones: Glucagon-like peptide-1 (GLP-1) and Glucose-dependent insulinotropic polypeptide (GIP). Here’s a breakdown of their individual roles and how Mounjaro leverages them:
Glucagon-like peptide-1 (GLP-1)
- Natural Role: Produced in the gut after eating, GLP-1 helps regulate blood sugar levels in various ways. It stimulates the pancreas to release more insulin (the hormone responsible for transporting sugar from the bloodstream into cells for energy). Additionally, GLP-1 slows down the emptying of food from the stomach, promoting feelings of fullness and reducing sugar absorption.
- Mounjaro’s Action: By mimicking the effects of natural GLP-1, Mounjaro enhances insulin production and slows down digestion, leading to better blood sugar control.
Glucose-dependent insulinotropic polypeptide (GIP)
- Natural Role: Also released from the gut after eating, GIP works alongside GLP-1 to further stimulate insulin secretion from the pancreas. It additionally plays a role in fat storage and insulin sensitivity.
- Mounjaro’s Action: By targeting GIP receptors, Mounjaro further amplifies the insulin-boosting effects and potentially influences fat metabolism, contributing to weight management benefits observed in studies.
In essence, Mounjaro acts like a double agent, targeting both GLP-1 and GIP pathways to achieve a more comprehensive approach to managing blood sugar and potentially aiding in weight loss and weight management. This dual action is what sets Mounjaro apart from traditional GLP-1 receptor agonist medications.
Clinical Studies and Weight Loss Results of Mounjaro
Mounjaro’s approval for weight management is based on several clinical trials demonstrating its effectiveness. Here’s a breakdown of some key studies and their weight loss findings:
Clinical Studies
The most prominent studies include the SURMOUNT program, a series of phase III clinical trials involving thousands of participants with overweight or obesity. The trial involved over 4,000 participants with obesity (BMI ≥ 30 kg/m²) or overweight (BMI ≥ 27 kg/m²) with weight-related comorbidities. These trials compared the effects of different Mounjaro doses to a placebo alongside lifestyle changes.
Weight Loss Results
The trials reported significant weight loss in participants receiving Mounjaro compared to the placebo group.
- These studies involved participants who received Mounjaro alongside a reduced-calorie diet and increased physical activity program over 72 weeks (approximately 1.5 years).
- The results were significant:
- Average Weight Loss: Compared to a placebo group, individuals using Mounjaro achieved an average weight loss ranging from 15% to 22% of their initial body weight.
- Percentage of Participants Losing Weight: Over 85% of participants taking Mounjaro lost at least 5% of their body weight, compared to only 35% in the placebo group.
What is the clinical criteria to obtain the Mounjaro injection?
The clinical criteria for obtaining a Mounjaro injection in the UK will depend on whether you’re seeking it for:
- Type 2 Diabetes Management:
- Diagnosis of Type 2 Diabetes: You must have a confirmed diagnosis of type 2 diabetes mellitus.
- Ineligibility or Intolerance to Metformin: If you cannot tolerate metformin, the first-line medication for type 2 diabetes, Mounjaro may be considered.
- Unsatisfactory Blood Sugar Control: If your blood sugar levels remain uncontrolled with existing medications, Mounjaro might be prescribed in combination with them.
- Weight Loss / Management:
Body Mass Index (BMI) Criteria: Mounjaro is currently approved for weight management in specific adult populations based on BMI:
- Obesity (BMI ≥ 30 kg/m²): Individuals classified as obese can potentially qualify for Mounjaro.
- Overweight (BMI 27-30 kg/m²) with Comorbidities: Adults who are overweight but have weight-related health problems like prediabetes, high blood pressure, or sleep apnea may be considered for Mounjaro alongside lifestyle changes.
What are the dosages and colours of Mounjaro?
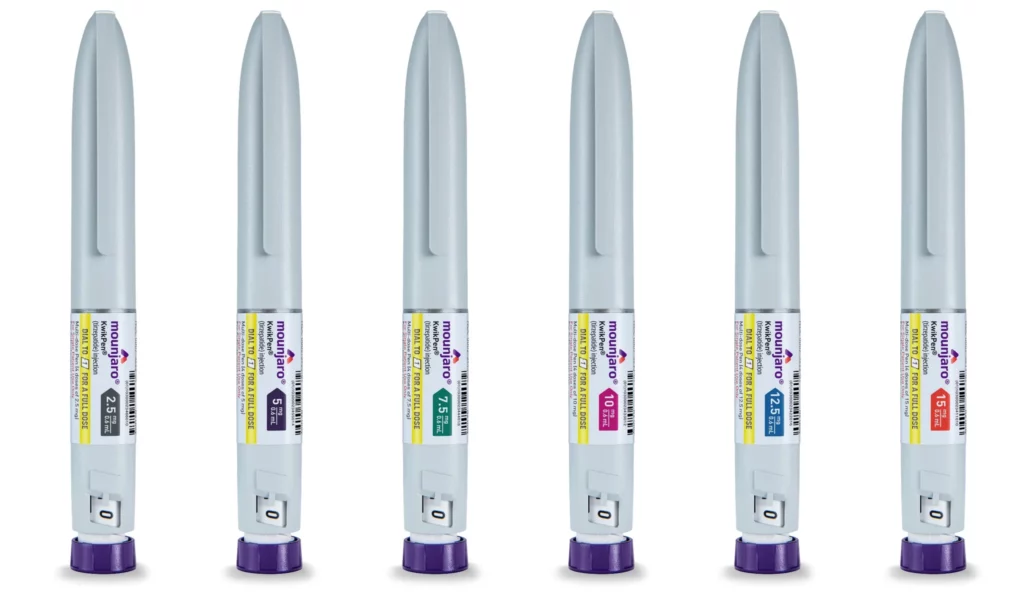
Mounjaro comes in a single pre-filled injection device called the Mounjaro KwikPen. However, the KwikPen delivers different dosages of the medication depending on the colour of the pen:
- Dosage: Mounjaro KwikPen is available in: 2.5mg, 5mg, 7.5mg, 10mg, 12.5mg, and 15mg.
- Colour: Each Mounjaro KwikPen has a distinct colour associated with the dosage it delivers:
- Grey Pen: Delivers 2.5mg of tirzepatide (the starting dose)
- Purple Pen: Delivers 5mg of tirzepatide
- Green Pen: Delivers 7.5mg of tirzepatide
- Pink Pen: Delivers 10mg of tirzepatide
- Blue Pen: Delivers 12.5mg of tirzepatide
- Orange Pen: Delivers 15mg of tirzepatide (the highest available dose)
It’s crucial to remember that the appropriate dosage of Mounjaro will be determined by your healthcare professional after considering individual factors like blood sugar control (for type 2 diabetes) or weight loss goals. Clinicians will also monitor your response and adjust the dosage as needed over time.
How much weight can you lose with Mounjaro?
Mounjaro has shown promising results in clinical trials for weight loss, but it’s important to understand the potential range and manage expectations. Here’s a breakdown:
- Average Weight Loss: Studies observed an average weight loss of 15% to 22% of initial body weight after 1.5 years (72 weeks) when Mounjaro was combined with a healthy diet and exercise plan.
- Individual results may vary: This is just an average, and your weight loss experience with Mounjaro could be different. Factors like your starting weight, body composition, genetics, and adherence to lifestyle changes can all influence your results.
What are the side effects of Mounjaro?
Mounjaro, like most medications, can cause side effects. While not everyone experiences them, it’s important to be aware of the potential ones. Here’s a breakdown of the most common side effects associated with Mounjaro:
Gastrointestinal Issues: These are the most frequent side effects and tend to occur more often when starting Mounjaro or increasing the dosage.
- They may include: Nausea, Vomiting, Diarrhea, Constipation, Abdominal pain, Indigestion (upset stomach), Belching, Flatulence.
- Decreased Appetite: Some people may experience a reduced appetite, which can contribute to weight loss efforts.
- Injection Site Reactions: Mild pain, redness, or swelling at the injection site are possible.
Less Common or Serious Side Effects:
- Low Blood Sugar (Hypoglycemia): This is more likely if you take Mounjaro with other medications that lower blood sugar, such as insulin. It’s crucial to monitor your blood sugar levels as directed by your healthcare professional.
- Pancreatitis (Inflammation of the Pancreas): This is a serious but rare side effect. Symptoms include severe upper abdominal pain that may radiate to your back. Stop taking Mounjaro and seek immediate medical attention if you experience this.
- Gallstones: Mounjaro may increase the risk of gallstones in some individuals.
Important Considerations:
- Severity and Duration: Most side effects associated with Mounjaro are mild and temporary, often subsiding within a few weeks as your body adjusts to the medication.
- Individual Experience: Not everyone will experience these side effects, and some people may tolerate Mounjaro very well.
Seek Support:
- Talk to Your Healthcare Professional: Discuss any potential side effects and your medical history with your clinician before starting Mounjaro. They can assess your individual risk factors and advise you on managing potential side effects.
- Report Side Effects: If you experience any side effects, especially if they are severe or persistent, be sure to report them to your clinician. They can adjust your dosage or recommend alternative medications if necessary.
How long does Mounjaro side effects last?
The duration of Mounjaro UK side effects can vary depending on the specific side effect and your individual response to the medication.
Most Common Side Effects: The common side effects associated with Mounjaro, particularly gastrointestinal issues like nausea, vomiting, diarrhea, and constipation, tend to be temporary. They often occur more frequently when you first start taking Mounjaro or when your clinician increases your dosage. These side effects may subside within a few days or weeks as your body adjusts to the medication.
Several factors can influence how long you experience side effects, including:
- Starting Dosage: Lower starting doses (2.5mg) are more likely to cause milder and shorter-lived side effects compared to higher doses.
- Severity of Side Effects: More severe side effects, although less common, may take longer to resolve and may require adjustments to your dosage or medication regimen.
- Individual Response: Everyone’s body reacts differently to medications. Some people may experience side effects for a shorter duration compared to others.
If side effects persist beyond a few weeks, discuss them with your healthcare professional. They may recommend strategies to manage them, such as adjusting your dosage, taking medication with food, or using over-the-counter medications to alleviate symptoms like nausea or heartburn.
Who can use Mounjaro?
Mounjaro isn’t suitable for everyone in the UK. Here’s a breakdown of who can potentially benefit from it and who may not be a good candidate:
- Potential Candidates:
- Adults with Type 2 Diabetes
- Adults for Weight Management
- People Who May Not Be Suitable:
- Certain Medical Conditions: Individuals with a history of pancreatitis (inflammation of the pancreas) or a type of tumour called medullary thyroid carcinoma (MTC) are not recommended to use Mounjaro.
- Pregnancy and Breastfeeding: Mounjaro is not recommended for pregnant or breastfeeding women due to the potential risks to the developing baby.
- Allergic Reactions: People with a known allergy to Mounjaro or its ingredients should avoid it.
How to inject, store and handle Mounjaro pens?
Mounjaro comes in a pre-filled injection. Here’s a breakdown of the injection process:
Before You Begin:
- Wash your hands: Thoroughly wash your hands with soap and warm water for at least 20 seconds to prevent infection.
- Gather supplies: You’ll need the Mounjaro KwikPen, alcohol wipes, and a sharps container for disposal.
- Inspect the pen: Check the expiration date on the pen label and ensure the liquid is clear and colorless. Don’t use the pen if it’s expired, damaged, or the liquid is discolored.
Injection Steps:
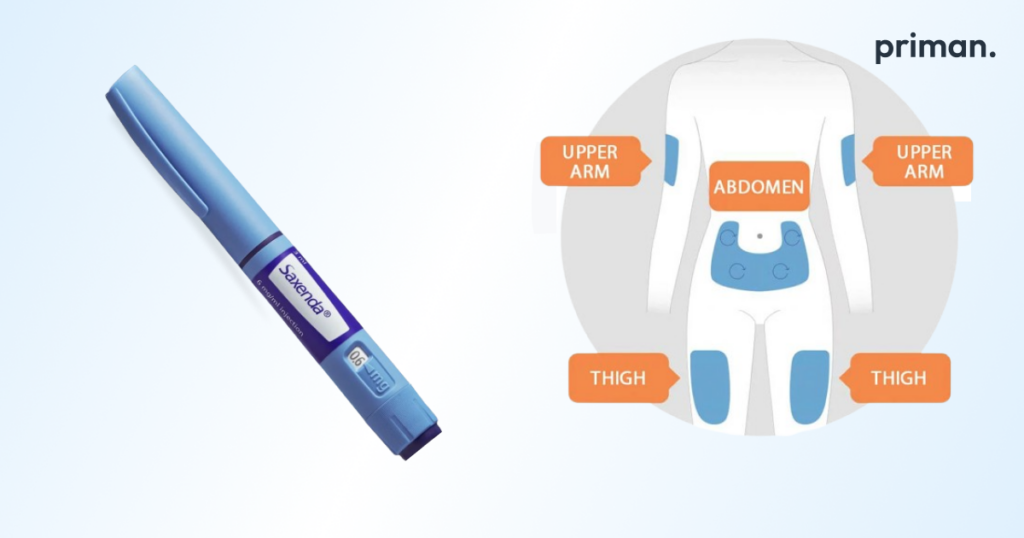
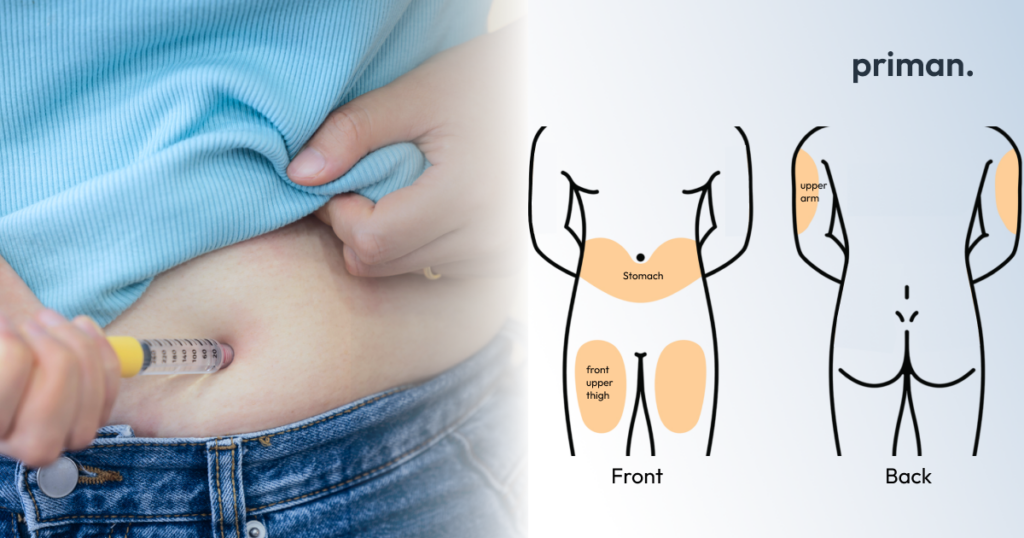
- Choose an injection site: You can inject Mounjaro in your stomach area (abdomen), thigh, or the back of your upper arm. Rotate injection sites with each injection to avoid irritation. Avoid injecting into areas with moles, scars, or redness.
- Clean the injection site: Use an alcohol wipe to clean the chosen injection site. Let the area dry completely before injecting.
- Unlock the pen: Follow the instructions on the pen to unlock the injection mechanism (refer to the Mounjaro patient information leaflet or consult with your doctor if needed).
- Hold the pen: Grasp the pen firmly with the needle pointing downwards.
- Inject and hold: Insert the needle straight into the cleaned injection site and press the injection button firmly. Hold the button down for at least 10 seconds until you hear a click, indicating a complete injection.
- Withdraw the needle: Remove the needle from the injection site straight up and discard it safely in a sharps container.
After Injection:
- Dispose of the pen: Do not reuse the Mounjaro KwikPen. Dispose of it safely in a designated sharps container after injecting the entire dose.
- Gently press the injection site: You may press gently on the injection site with a clean gauze pad after removing the needle. Don’t rub the area.
Storing and Handling Mounjaro Pen:
- Store in the refrigerator: Keep your Mounjaro KwikPen in the refrigerator (between 36°F to 46°F or 2°C to 8°C). Do not freeze the pen.
- Travel considerations: If you need to travel with your Mounjaro pen for short periods (up to 21 days), you can store it at room temperature (up to 86°F or 30°C).
- Protect from light: Keep the pen in its original carton to protect it from light.
- Keep out of reach of children and pets: Store the pen in a safe place out of the sight and reach of children and pets.
Important Tips
- Follow your healthcare professional’s instructions: Always follow the specific instructions provided by your doctor for using the Mounjaro KwikPen.
- Don’t reuse needles: Never reuse a needle or share your pen with anyone else.
- Seek help if needed: If you are unsure about any aspect of injecting Mounjaro, don’t hesitate to ask your clinician or pharmacist for help.
- Do not remove the needle cap from the pen until you are ready to inject.
- Never inject Mounjaro into a vein or muscle.
- If you miss a dose of Mounjaro, don’t inject a double dose. Just continue with your regular dosing schedule.
Where to buy Mounjaro in the UK?
Mounjaro isn’t available for purchase like typical over-the-counter weight loss medications.
- Prescription Requirement: Mounjaro requires a prescription from a licensed healthcare provider who can assess your suitability based on National Institute for Health and Care Excellence (NICE) criteria. So you cannot buy it directly from a pharmacy or online retailer without a doctor’s approval.
- Not Over-the-Counter: Unlike some weight loss supplements or tablets, Mounjaro requires an assessment due to its potential side effects and targeted use based on specific criteria.
- Consultation: Connect with licensed healthcare providers who can evaluate your eligibility for Mounjaro. During the consultation, they will discuss your medical history, weight loss goals, and assess if Mounjaro aligns with NICE criteria.
Empower Your Weight Loss Journey: Start with Priman Online Assessment!
References
Lapid, N. (ed.) (2023) More weight loss with Mounjaro than ozempic: Data analysis, Medscape.
Samms, R. J., Coghlan, M. P. and Sloop, K. W. (2020) “How may GIP enhance the therapeutic efficacy of GLP-1?,” Trends in endocrinology and metabolism: TEM, 31(6), pp. 410–421. doi: 10.1016/j.tem.2020.02.006.
Jastreboff, A. M. et al. (2022) “Tirzepatide once weekly for the treatment of obesity,” The New England journal of medicine, 387(3), pp. 205–216. doi: 10.1056/nejmoa2206038.
Wadden, T. A. et al. (2023) “Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial,” Nature medicine, 29(11), pp. 2909–2918. doi: 10.1038/s41591-023-02597-w.
Lilly.com. Available at: https://www.lilly.com/news/media/media-kits/mounjaro
Burger, L. (2024) Britain sees launch of Lilly’s weight-loss drug Mounjaro, Reuters.com. Available at: https://www.reuters.com/business/healthcare-pharmaceuticals/britain-sees-launch-lillys-weight-loss-drug-mounjaro-2024-02-14/
Healthcare products Regulatory Agency (2024) Four-dose Mounjaro “KwikPen” approved by MHRA for diabetes and weight management, Gov.uk. Available at: https://www.gov.uk/government/news/four-dose-mounjaro-kwikpen-approved-by-mhra-for-diabetes-and-weight-management
Jastreboff, A. M. et al. (2022) “Tirzepatide once weekly for the treatment of obesity,” The New England journal of medicine, 387(3), pp. 205–216. doi: 10.1056/nejmoa2206038.
Mounjaro (tirzepatide) (2024) Diabetes UK. Available at: https://www.diabetes.org.uk/guide-to-diabetes/managing-your-diabetes/treating-your-diabetes/tablets-and-medication/glp-1/mounjaro
Priyan, V. (2023) MHRA grants authorisation for Lilly’s weight loss drug Mounjaro, Pharmaceutical Technology. Available at: https://www.pharmaceutical-technology.com/news/mhra-lilly-mounjaro/